
Melis Caliskan
- Administration
- melis.caliskan@somi-medical.com
- +49 7231 56619-270
The cleaning, assembly and packaging of your products takes place in our clean rooms DIN EN ISO 14644, class 7. Reliable quality management is the basis for our business. We are certified according to DIN EN ISO 13485.

In accordance with the product characteristics, we work with our customers to develop cleaning processes that meet the normative and legal requirements for a medical device.

We are happy to carry out a wide range of assembly activities for you, both in the class 7 cleanroom and in the grey area.
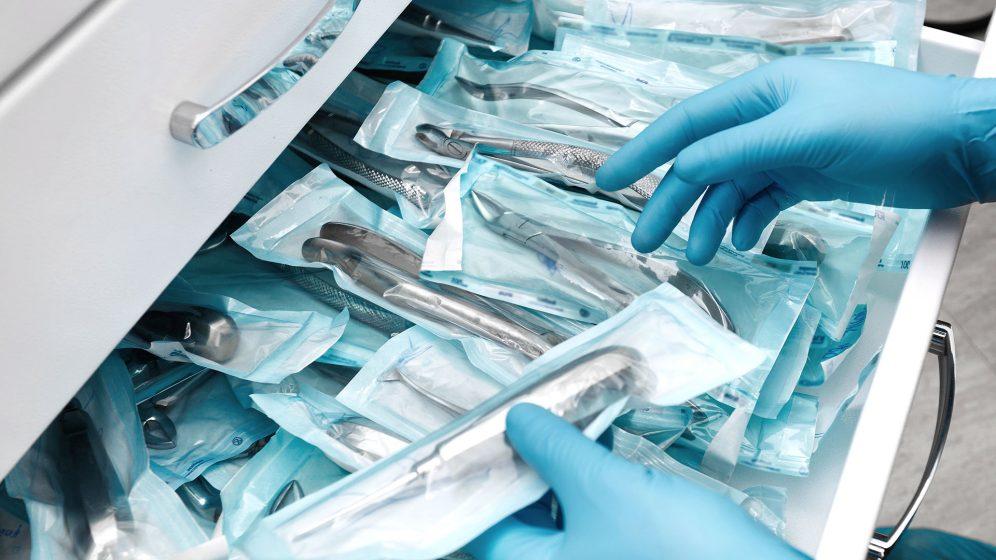
For the entire spectrum of your products, we realize the appropriate packaging according to your requirements and specifications – regardless of whether it is sterile or non-sterile packaging.
We support you not only in the selection and implementation of sterilization validation, but also in advance in defining the appropriate sterilization process for your product.

